
There is more mixing involved, but the atoms of the gas go from being completely separated from each other to being closely packed with each other and the solvent. Entropy usually decreases when a gas dissolves in a liquid or solid.After mixing, they are completely interspersed within each other. Entropy usually increases when a liquid or solid dissolves in a solvent.īefore mixing, the solute and solvent are completely separated from each other.Two more patterns emerge from considering the implications of the first three. Therefore, the stronger bond will cause less disorder and less entropy. If you think of ionic bonds as springs, a stronger bond will hold the ions in place more than a weaker bond. Entropies of ionic solids are larger when the bonds within them are weaker (columns 3 and 4).The chains of polyethylene, while more complex than ethylene, results in stronger intermolecular forces that keeps it in a different state of matter at. for ideal solutions, where R is the gas constant and xi is the molar. A gas has a much greater entropy than a solid, so ethene would have the greater standard molar entropy at 298 K. High-entropy and medium-entropy alloys are presumed to have a configurational. Large, complicated molecules have more disorder because of the greater number of ways they can move around in three-dimensional space. At 298K (25 Celsius), ethene (ethylene) is a gas while polyethylene is a solid. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2).The atoms in gases are far apart from each other, so they are much more disordered than either liquids or solids.
#Molar entropy free#
The atoms in liquids are still close together but they are free to move around with respect to each other, so they are more disordered. On the nanoscale level, the atoms in solids are constrained to one position they can only vibrate around that position. Therefore, q and DS are both positive and the liquid or gas has more entropy than the solid or liquid. This can be predicted from equation (1): heat must be put into substances to convert them from solid to liquid or liquid to gas. The entropies of gases are much larger than those of liquids, which are larger than those of solids (columns 1, 3, and 4).
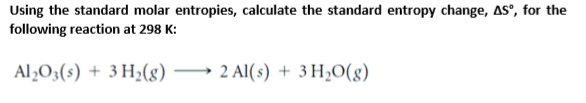
Some patterns emerge when these values are compared. (eds.In the table below are some excerpts from the Thermodynamics Table. Chemical Engineering Research Information Center.
Properties of aqueous methanol solutions ĭata obtained from Lange's Handbook of Chemistry, 10th ed. Note that the form of this formula as given is a fit to the Clausius–Clapeyron equation, which is a good theoretical starting point for calculating saturation vapor pressures: Here is a similar formula from the 67th edition of the CRC handbook. Uses Antoine's equation: P m m H g = 10 7.87863 − 1473.11 230.0 + T from Lange's Handbook of Chemistry 10th ed. Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. Thermodynamic properties Phase behaviorħ0.8–90.5 J/(mol K) (at −97.6 to 64.7 ☌) Baker and Loba Chemie Structure and properties Structure and properties It is highly recommended that you seek the Safety Datasheet ( SDS) for this chemical from a reliable source such as SIRI, and follow its directions. The handling of this chemical may incur notable safety precautions. This page provides supplementary chemical data on methanol.


 0 kommentar(er)
0 kommentar(er)
